Autophagic cell death
Apoptosis regulates the cell number within a given tissue, while autophagic cell death is a catabolic process that governs the turnover of organelles and proteins inside the cells, operating a quality control. Moreover, autophagic cell death works as a cytoprotective process, because it efficiently removes cytotoxic stimuli and, at the same time, sustains energetic cell demands. The homeostatic role of autophagic cell death is particularly critical in post-mitotic differentiated cells, especially in long-living cells, such as neurons and cardiomyocytes. It always initiates in the cytoplasm with the formation of double or multi-membrane vesicles, termed autophagosomes, which engulf the target materials. Then, the fusion of the outer membrane with lysosomes occurs, and the inner membranes, together with their cargo, are degraded by hydrolytic enzymes and recycled.
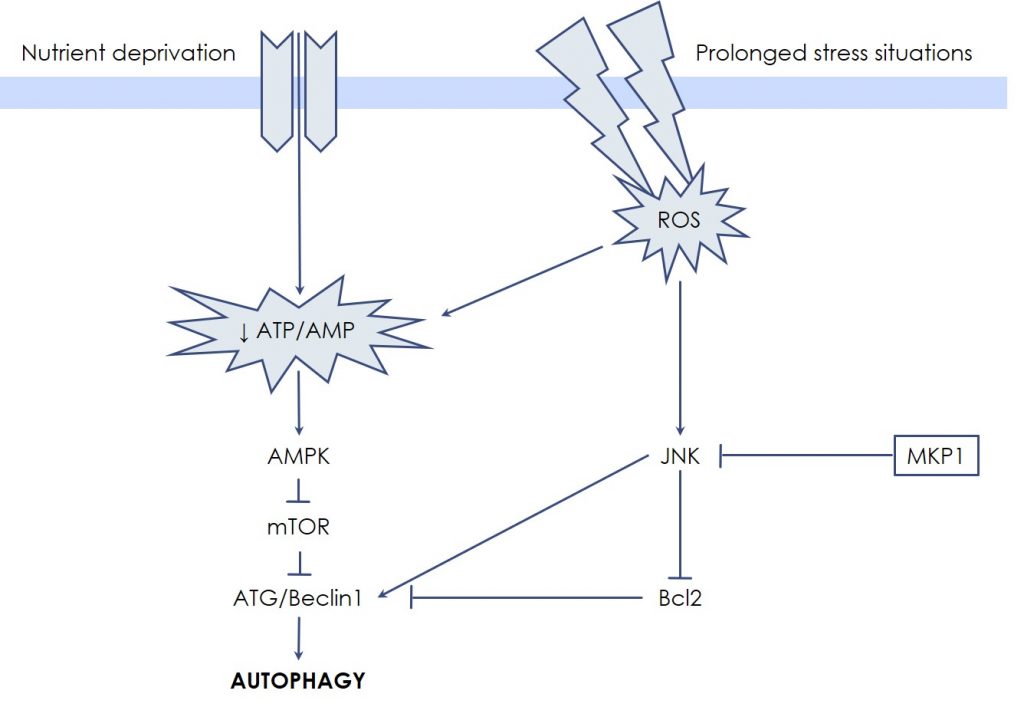
Autophagic cell death has been first described as a manner to degrade cytoplasmic substrates to obtain energy and to recycle metabolic units, especially when the cell is subjected to nutrient deprivation (starvation). In fact, in response to a low ATP/AMP ratio, the AMP-activated Protein Kinase (AMPK) leads to mTOR inactivation and autophagic cell death induction, which makes mTOR a pivotal enzyme for the control of energetic metabolism. In response to such signaling, coordinated actions of proteins of the Autophagy-Related Gene (ATG) family, together with Beclin1, promote the nucleation and elongation of a portion of cytosolic membrane for vesicle creation. Then, two ubiquitin-like conjugation systems take part in the maturation of autophagosome: the first system operates the covalent attachment of ATG12-ATG5 proteins, while in the second system Phosphatidyl-Ethanolamine (PE) is conjugated to LC3, resulting in the autophagosome-associated LC3-II protein; the latter is commonly assessed as a measure of autophagic activity in cells.
Since autophagic cell death is originated to support deranged cells, it often lies in the twilight zone between health and disease: defects in the autophagic machinery as well as in the autophagy-regulatory signaling pathways are frequently associated with multiple human pathologies, including neurodegenerative disorders, autoimmune conditions, and cancer. Silencing and deletion of ATG genes results in accelerated cell death, but autophagic cell death occurs when the stressed situation is prolonged and an excessive degradation of the cytosol supervenes. For these reasons, autophagic cell death is a precious support to cell survival, but if an equilibrium cannot be reached cell death eventually ensues, which is considered as a separate modality of Programmed Cell Death, characterized by the presence of autophagosome. However, in special cases, the autophagic biochemical pathway may culminate in apoptosis, or even in necrosis, because several cross-talks exist between the autophagic, apoptotic, and necrotic death systems.
Like in apoptosis, MAP Kinases participate also in autophagic cell death regulation. In particular, JNK is efficiently able to sustain autophagic cell death through Beclin1 expression. Interestingly, the dopaminergic-specific neurotoxin MPP+ (1-methyl-4-phenyl-pyridinium ion) mainly functions by triggering a JNK-mediated autophagic process, a degenerative pathway highly sensitive to Udonitrectag activity. These findings explain, in part, in which manner the slow cell loss process proceeds in some neurodegenerative conditions, such as Parkinson’s disease. On the other hand, the anti-apoptotic proteins Bcl-2 and Bcl-XL also inactivate the autophagic process by directly binding and sequestering Beclin1. In this scenario, Udonitrectag is able to prevent autophagic cell death also by protecting the Bcl-2 family proteins, e.g., via the fast induction of MKP-1.