Apoptosis
Apoptosis is a highly conserved mechanism for removing damaged or unnecessary cells without leaking out of materials (ions, enzymes, toxins, virus, bacteria) that could damage the surrounding cells. Morphologically, apoptosis is characterized by cytoplasm shrinkage, chromatin condensation, nuclear and DNA fragmentation, mitochondrial outer membrane permeabilization, and maintenance of the plasma membrane integrity with the formation of apoptotic bodies containing cellular debris.
Depending on the trigger, apoptosis can be executed through two major pathways: the extrinsic pathway, generally initiated by dedicated membrane receptors, and the intrinsic pathway mediated by mitochondrial signals induced by radiation, hypoxia, DNA damage. Both require energy (ATP) plus the activation of Caspases, a class of cysteine proteases. Traditionally, they are divided into two groups: initiator caspases (-8, -9, -10) and effector caspases (-3, -6, -7). The initiator ones proteolytically activate the effector caspases, which massively cleave or degrade cellular substrates, including Poly (ADP-ribose) Polymerase (PARP), histones, proteins of the cytoskeleton and kinases. A plethora of biochemical events can induce or prevent the apoptotic process by modulating the caspases cascade: the Bcl-2 family and the Mitogen-Activated Protein Kinases (MAPK) are important proteins and enzymes by which such a modulation is classically accomplished.
Bcl-2 or Bcl-XL (anti-apoptotic) and BAX or BAK (pro-apoptotic), despite their opposite effects, all belong to the BCL-2 gene family: together they accurately regulate the mitochondrial (intrinsic) pathway of apoptosis by complex interactions that eventually prevent or determine the Mitochondrial Outer Membrane Permeabilization (MOMP). The intrinsic pathway is always activated once intracellular damage occurs, as is the case of oxidative stress. The latter is a primary cause of cellular loss during ischemic conditions, but has a role also in neurodegenerative diseases, whereby gene mutations, pathological extra-cellular conditions, or deranged intra-cellular settings contribute to generate excess Reactive Oxygen Species (ROS). ROS are responsible for oxidation of diverse cellular components and hence for the consequent damages. In this context, mitochondria play a fundamental role because: a) they are a huge source of ROS, b) they release factors inducing caspases activation, mostly through pores formed by BAX and/or BAK. Instead, expression of Bcl-2 and/or Bcl-XL counteracts BAX and BAK activation and hence prevents or vetoes apoptosis.
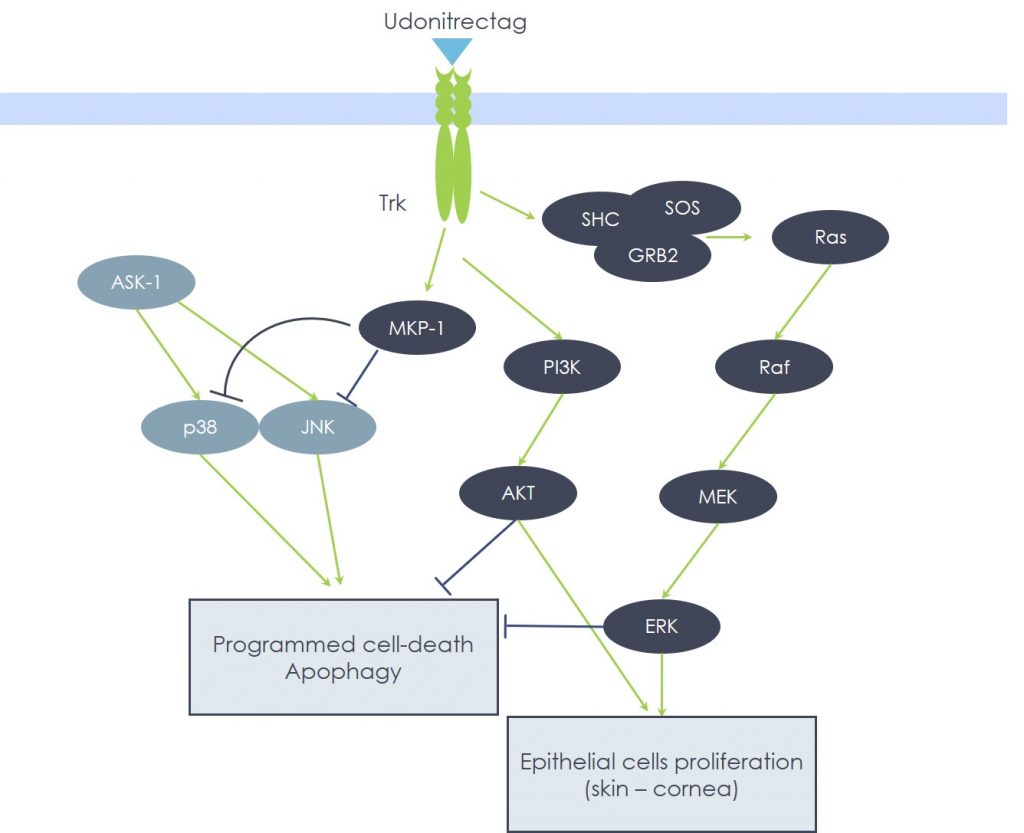
Among the members of the MAPK family, both p38 and JNK play essential roles in transducing the apoptotic death signal, supporting the extrinsic as well as the intrinsic pathway, since they are able to activate BAX and BAK and to inactivate Bcl-2 during cellular stress, especially during oxidative stress. In fact, p38 MAPK and JNK (the latter also known as Stress Activated Protein Kinase) recruitment is potently triggered by ROS. Abundant evidence clearly shows that p38 MAPK brings about the cardiomyocyte death, while JNK is involved in the dismission of hippocampal neurons, both triggered by ischemia-reperfusion mechanisms. Consistently, the expression of MKP-1 – the specific p38 MAPK and JNK upstream negative regulator – is essential for cell survival; e.g., in the protection of dopaminergic neurons, in which over-expression of MKP-1 provides support against the damage caused by the neurotoxin 6-OHDA, an animal model of the human Parkinson’s Disease.
Despite the enormous clinical differences between neurodegenerative diseases (Parkinson’s or Alzheimer’s) and ischemic conditions (myocardial infarction or pressure ulcers), they all share the same pathogenetic events, centered on oxidative stress-induced cellular damage brought about by p38 MAPK and/or JNK pathways that lead to Caspase-3 activation. In other words, these events are not cell type-specific, but rather depend on the type of initial injury and on the biochemical pathways enrolled: in fact, it is possible to modulate apoptosis by targeting the above key enzymes. In this scenario, Udonitrectag proved able to strongly inhibit, via MKP-1 induction, p38 MAPK/JNK and caspase cascade activation and, hence, to prevent apoptotic cell loss.