Programmed and non-programmed cell death in the pathogenesis of diseases
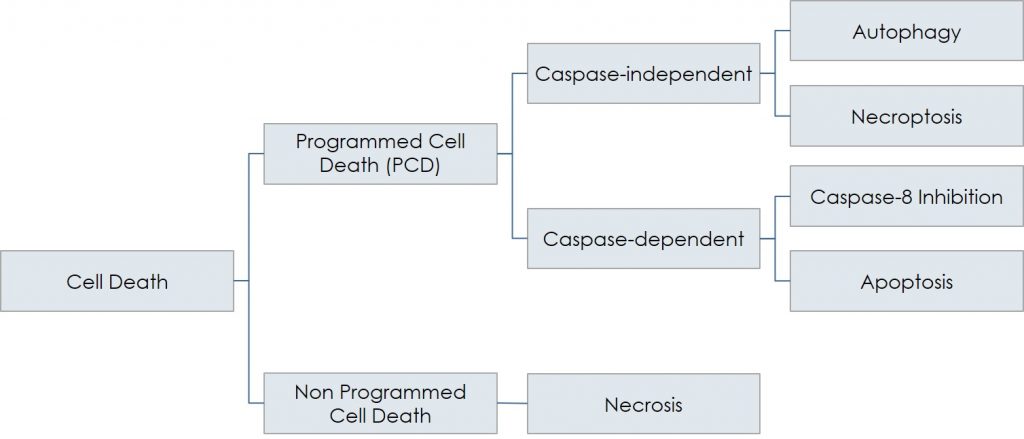
Schematically, necrosis (non-programmed cell death) is the opposite of programmed cell death, PCD, which includes caspase-dependent (apoptosis) as well as caspase-independent death (autophagy and necroptosis). Besides their involvement in various physiological events, all the different forms of PCD play a fundamental role in the pathogenesis of numerous conditions, including neurodegenerative (Parkinson’s, Alzheimer’s, retinitis) and ischemic (i.e., stroke, myocardial infarction) diseases, all arising when a group of cells are damaged and, consequently, die through controlled processes:
- Ischemic diseases: in cardiac infarction or in stroke, occlusion of an artery results in an early, massive necrotic cell death, due to an acute, intolerable deprivation of oxygen and nutrients: the so-called ischemia. Currently, the standard treatment for these acute events is aimed to removal of the obstruction to allow reopening of the vessel, also using surgical procedures; if successful, these efforts produce the so-called reperfusion of the ischemic tissue. As in general a prolonged ischemia determines a proportionally severe necrosis, reperfusion is always pursued; but it doesn’t come at no price. In fact, despite its efficacy in blocking necrosis, together with nutrients and oxygen reperfusion always brings oxidative stress and inflammation, which give rise to the apoptotic and necroptotic events, determining what is known as the ischemia-reperfusion injury. The latter is mainly caused by the Reactive Oxygen Species (ROS), effectors molecules that bring about the oxidative stress. Nevertheless, this process is not totally irreversible, as it is possible to rescue the dying cell by providing and/or enhancing adequate pro-survival stimuli, possibly in the initial phase of the process.
- Neurodegenerative diseases: their etiology is largely unknown and their pathogenesis is really complex, but all share one trait: the progressive loss of cells that die by PCD. The kinetics of such cell death is unclear, since it takes many years to fully develop, but apoptosis, autophagy, and necroptosis, variously intermingled, are found in quite all neurodegenerative conditions, from Parkinson’s disease to retinal degeneration. Thus, it is evident that a drug, able to slow down or even arrest the PCD events, is highly sought.
Currently, pharmacological treatments able to provide metabolically deranged cells with an effective anti-apoptotic/pro-survival stimulus are not available. Nonetheless, it is known that such stimuli are physiologically conveyed by certain cytokines, known as growth/survival factors; among them, the neurotrophins, a family of proteins that comprises Nerve Growth Factor (NGF), Brain-Derived Neurotrophic Factor (BDNF), Neurotrophin (NT)-3, and NT-4/5. Increasing evidence indicates that these cytokines may bear particular importance, as their specific receptors – the Trk family of surface molecules – are expressed by a very large number of cell types, in a much wider fashion than previously imagined, suggesting that neurotrophins critically participate in the overall control of the death/survival divide. Such evidence underscores the opportunity of exploiting the biologic signal of neurotrophins for pharmacologic purposes in many different clinical settings. However, the development of a drug based on a recombinant neurotrophin protein has been attempted in the past with little success, and often eventually discarded, essentially because of critical, prohibitive pharmacokinetics problems. Udonitrectag overcomes these problems and offers an unprecedented strategy to treat the diseases based on loss of viable cells due to PCD (apoptosis, autophagy, and necroptosis).